Last year, UPM Biomedicals hosted its 9th annual conference. The theme of the conference was “The future of 3D cell culture: from research to treatments”. In this article series, we’re highlighting some of the key takeaways from the informative presentations shared by industry and academia representatives at the conference. In the previous article, we looked at the benefits of animal-free 3D cell models in drug discovery and beyond. In this third and final article of the series, we focus on drug toxicology screening, and how 3D cell models help optimize the drug discovery process.

Toxicology is a discipline at the epicenter of the preclinical drug discovery and development pipeline. Screening compounds and interrogating their impacts on cell viability is a key starting point when looking to narrow many thousands of candidate compounds down to just a few promising leads. The scope of toxicology applications in drug discovery reaches far beyond probing compound safety – it can also uncover mechanistic information about a drug, identify dangerous off-target effects, inform drug dosing and much more besides.
In our second UPM conference article, we highlighted some of the challenges presently faced by the pharmaceutical industry, particularly in translating pre-clinical data gained through in vitro testing and high throughput screening (HTS) into success in human trials. This translational gap indicates a failure of preclinical models to accurately predict human responses, and contributes to 9 out of 10 candidate drugs failing once they reach clinical trials, despite taking on average 12 years and $1bn to get there.
So, what can be done to address this high failure rate? Stakeholders across the pharmaceutical industry and academia are seeking to bridge the gap by developing preclinical models that more closely represent the patient populations that they are trying to treat. In particular, the emergence of 3D cell culture has been a game-changer in the field. Unlike the 2D cultures that have been leveraged for toxicology screening for close to a century, 3D models can closely replicate the architecture, cellular heterogeneity and various physiological features of human tissues.
The ability to generate these 3D models from human stem cells, or directly from patients as ex vivo models, has opened up a world of possibilities in preclinical toxicology screening. Adopting more accurate 3D models in the preclinical phases promises to streamline drug discovery, optimize drug candidate selection and lower costs, while reducing the likelihood of late-stage drug failures and fostering the development of safer and more effective therapeutic agents.
3D models in toxicology screening
Toxicology screening in drug discovery often necessitates the evaluation of hundreds or thousands of compounds. As such, toxicology applications in drug discovery demand a 3D cell platform that balances physiological relevance (predictivity), scalability, compatibility with automation technology and cost-effectiveness. Striking this balance is crucial as increasing complexity and human physiological relevance in 3D models invariably brings higher costs, reduced scalability and poorer compatibility with HTS.
The standout 3D cell models for toxicology screening are spheroids. Spheroids are spherical aggregates of cells that form naturally when cultured in a non-adherent environment. They represent one of the most widely used 3D cell culture models in toxicology screening due to their simplicity, cost-effectiveness and ability to mimic the in vivo cellular environment more accurately than traditional 2D cultures. Unlike more complex organoid models, spheroid cultures do not aim to replicate the intricate cellular makeup or physiology of real organs. Instead, they offer a means of creating an advanced scalable 3D platform that can be extremely valuable in enhancing the predictive value of toxicology screens.

Spheroid culture techniques: ULA Plates vs. Hydrogels
Spheroid generation can be achieved through various methods, but by far the most common techniques employed are matrix-based culture and suspension-based cultures (i.e. ultra-low attachment (ULA) plates). Matrix-based culture involves embedding cells within a hydrogel matrix. Hydrogel matrices mimic the extracellular matrix found in tissues, providing structural support and promoting natural cell-cell and cell-matrix interactions. The hydrogel environment facilitates the formation of spheroids by allowing cells to migrate and aggregate naturally, forming compact, spherical 3D structures.

Hydrogels can differ broadly in their material composition, with many commercially available products derived from animal-based sources. Animal-derived hydrogels add complexity in preparation and handling, as well as spheroid harvesting for downstream analysis. Furthermore, they can bring unintended experimental effects from animal source contaminants. Opting for an animal-free matrix, like , enables smooth handling, easy retrieval and eliminates unwanted experimental effects.
Spheroids can also be generated in suspension e.g. using ULA plates. ULA plates have been designed to prevent cells from adhering to the surface, encouraging them to aggregate and form spheroids. Cells are seeded into the round-bottom wells, where they are left to naturally aggregate to create spheroids, thanks to the non-adhesive surface. This method is straightforward and commonly employed in HTS applications for its simpler workflow, cost-effectiveness and scalability.
However, spheroids generated in ULA plates may lack the structural and functional complexity of those formed in matrix-based cultures, as they do not have the same extracellular matrix support. Without a supporting matrix, spheroids in ULA plates may aggregate or settle unevenly, leading to variability in size and shape [1].
Miniaturization in toxicology screening: The Rise of 3D Hepatotoxicity Models
One of the most exciting advancements in 3D cell culture technology is the ability to miniaturize complex 3D models into a scalable platform amenable to large-scale toxicology screens. Miniaturization is critical when using primary tissue, allowing researchers to maximize the data yield from limited primary cell samples, a common challenge in preclinical testing. One of the most popular tissues to miniaturize in toxicology screening platforms is liver. Hepatocyte models are highly favorable for toxicity screening due to the liver's central role in drug metabolism and detoxification. As the primary organ responsible for metabolizing and clearing drugs from the body, the liver is often the first site of drug-induced toxicity.
Utilizing human primary hepatocytes in these models provide a more accurate representation of human liver function, and unlocks the ability to target specific patient populations, or to account for heterogeneity in responses between patients. Human hepatocytes only maintain their phenotype and functional characteristics for a limited time when cultured in 2D monolayers. 3D cell culture helps preserve their phenotype and functionality for longer periods. This extended viability in 3D cultures allows for more reliable and relevant toxicity assessments, making 3D hepatocyte models a highly promising platform for preclinical drug discovery.
The Nanoscale Drug Testing Project
One such project that is striving to miniaturize human hepatocytes to develop a drug toxicology screening platform, is the Nanoscale Drug Testing Project. Based in Sweden, this innovative collaboration between academic researchers, industry experts and solution providers aims to revolutionize preclinical drug testing using advanced 3D cell culture techniques.
At the heart of this project is the development of a primary human hepatocytes model based on mini-spheroids—tiny clusters of cells that maintain phenotypic traits of the human liver. These spheroids are generated using a microfluidic encapsulation device, which enables the formation of uniform, miniature spheroids composed of 60-100 cells each. The mini-spheroids are compatible with multi-well plate formats, enabling the generation of highly reproducible cultures for hepatotoxicity testing. Reproducibility is crucial for drug screening platforms because it ensures consistent and reliable results when assessing the safety and efficacy of new compounds.
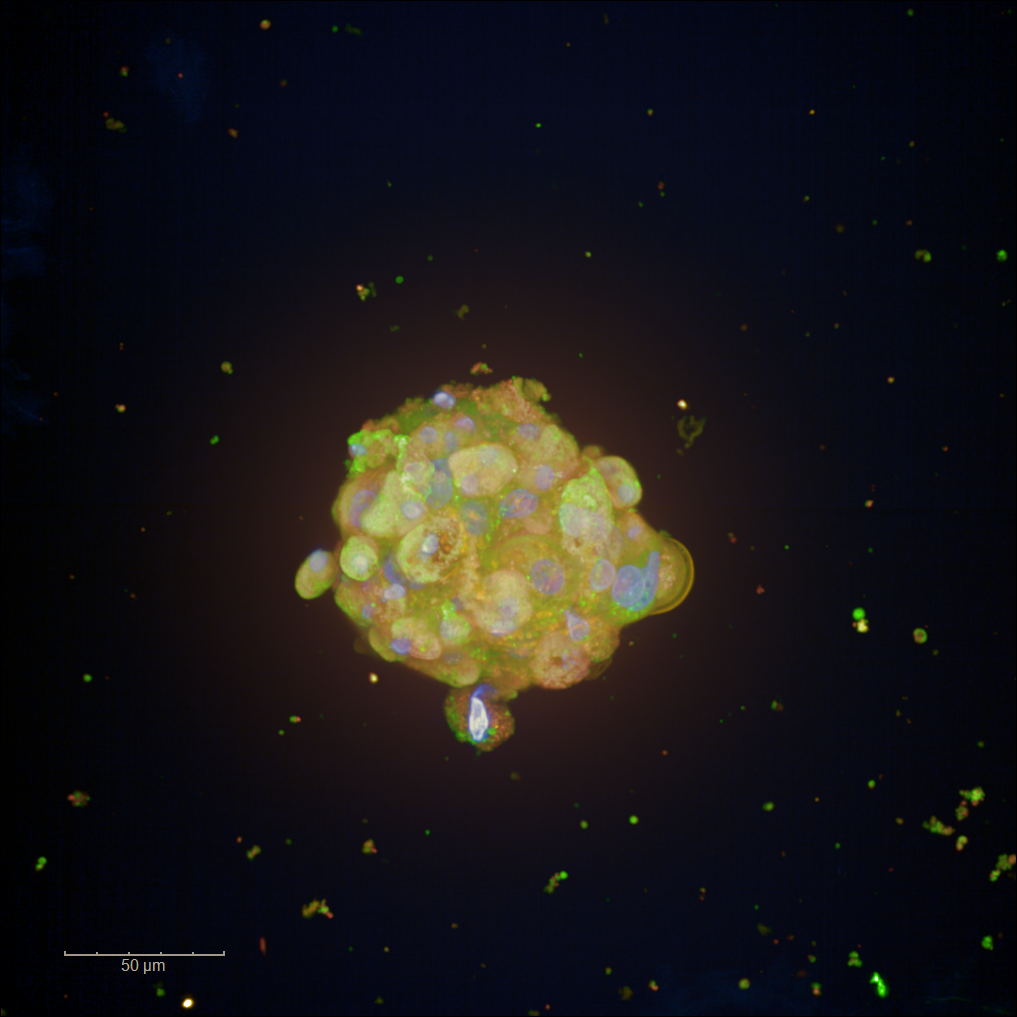
Figure 1: Image by Heshuang Qu/ Karolinska Institute and SciLifeLab, Sweden
This hydrogel scaffold prevents spheroids from aggregating to each other ensures uniform distribution, and allows for easy handling during automated high-throughput screening processes. By maintaining spheroid consistency and compatibility with automated dispensing, GrowDex® has helped to enhance the reproducibility and reliability of the mini-spheroid platform.
The Nanoscale Drug Testing Project demonstrates several key advantages of using spheroids for toxicology screening. Firstly, the physiological relevance of spheroids derived from primary tissue allows for more accurate prediction of drug effects and toxicity, bridging the gap between preclinical models and human physiology. Secondly, the miniaturized spheroid format is ideal for high-throughput screening, enabling the efficient testing of numerous compounds with reduced resource expenditure.
Since the primary human hepatocytes used in the mini spheroids retain their phenotypic traits, they respond to drugs in ways that more closely reflect patient responses. This enhanced predictive accuracy will be invaluable for identifying promising drug candidates earlier in the development process, potentially increasing the success rate of clinical trials and reducing the incidence of late-stage failures.
DIVA-Caps: a model for studying adipose tissue
Another major advantage of miniaturized 3D models in toxicology screening is the ability to generate models of specific human tissues involved in the disease that drug developers are seeking to treat. Mayoura Keophiphath, founder and CEO of French biotech Diva Expertise, highlighted this potential in an interesting conference talk about the development of a 3D human adipose tissue model known as DIVA-Caps.
Excessive fat mass accumulation in obesity can give way to a diverse range of metabolic disorders, increased risks of cardiovascular diseases, and in some cases, mortality. Addressing these issues, Mayoura and her team have developed an innovative in vitro model combining human intestinal cells with human adipose cells. This model is particularly relevant for screening botanical extracts and other compounds for their effects on adipose tissue metabolism, including lipid accumulation, thermogenesis and lipolysis.
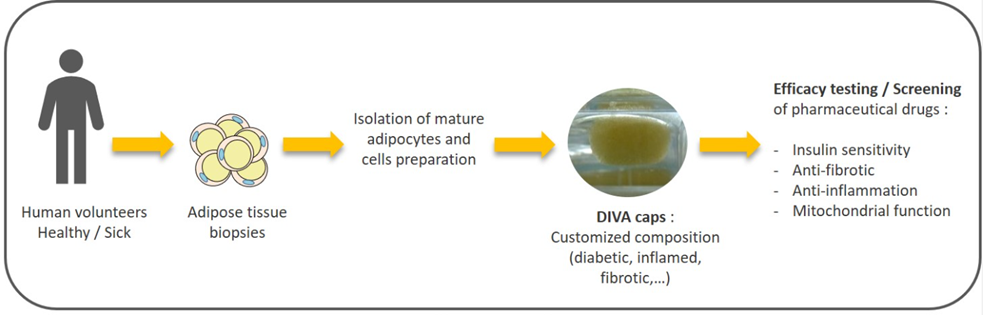
Figure 2: Generation of DIVA-Caps 3D adipose tissue models. https://www.diva-expertise.com/
Mayoura’s research focuses on a bi-phasic culture system that integrates Caco-2 cells, which mimic the intestinal epithelium, with human adipose cells. This model aims to replicate the intestinal passage of orally administered compounds and their subsequent effects on adipose tissue. By doing so, the DIVA-Caps model provides a more physiologically relevant platform for studying the interactions between ingested substances and adipose tissue, including toxicity, which is crucial for developing treatments for obesity-related disorders [2].
Regulatory New Approach Methodologies (NAMs) in Toxicology
Before a new drug can enter clinical testing, it must undergo an extensive array of toxicity tests to ensure its safety. Traditional toxicity testing has relied heavily on animal models, such as rats, mice and rabbits, to assess factors including chronic and acute systemic toxicity, local toxicity and irritation, genotoxicity, carcinogenicity and other toxic effects. Using in vivo models for this is not only ethically challenging, but also time-consuming, costly and provides poor predictive value.
In another insightful conference presentation, Tuula Heinonen of Tampere University discussed the evolving regulatory landscape surrounding the use of in vitro methods like spheroid platforms, often referred to as New Approach Methodologies (NAMs), in toxicity screening. Her talk highlighted the growing acceptance of these methods by major regulatory bodies, including the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA).
Both the EU and FDA have implemented measures to minimize animal testing and promote the use of in vitro methods. The EMA has been proactive in encouraging the adoption of the 3Rs (Replacement, Reduction, and Refinement) through its Innovation Task Force (ITF). Since September 2021, the EMA has emphasized that new drugs do not necessarily need to be tested on animals before human studies. This significant shift is aimed at accelerating the development of new therapies while reducing the ethical and financial burdens associated with animal testing.
Similarly, the FDA supports the use of non-animal methods to bring FDA-regulated products to market faster and to prevent products with increased toxicological risk from reaching the market. The U.S. Environmental Protection Agency (EPA) has also set ambitious goals to reduce animal testing by 30 percent by 2025 and eliminate it by 2035. The European Chemicals Agency (ECHA) under the REACH regulation mandates that animal testing should be a last resort, further underscoring the commitment to in vitro methods.
As a result of the renewed focus on reducing animal testing, the acceptance of NAMs by regulatory bodies has grown significantly. Validated in vitro tests, compliant with OECD guidelines, are now pivotal for safety assessments in various sectors, including drugs, biocides, pesticides, and industrial chemicals. So long as NAMs are demonstrated to be repeatable, reproducible and relevant, they can be harnessed in place of animal testing in preclinical drug development. This should give hope to researchers seeking to develop 3D models into toxicity screening platforms. The future of toxicology screening lies in these innovative in vitro methodologies that promise faster, safer and more ethical drug development.
A new dawn for toxicology screening

The 9th Annual UPM Biomedicals conference showcased some of the remarkable advancements and ongoing research in 3D cell culture technology, particularly in the realm of toxicology screening. The presentations highlighted the critical role that 3D cell models play in bridging the gap between in vitro testing and human clinical trials.
By offering more physiologically relevant environments, 3D models can significantly enhance the predictive accuracy of toxicology screens, reduce late-stage drug failures, and ultimately accelerate the development of safer and more effective therapeutics. The evolving regulatory landscape reflects a growing acceptance and encouragement of NAMs by major regulatory bodies. This shift towards minimizing animal testing and promoting in vitro methods aligns with the ethical, financial and scientific goals of the pharmaceutical industry.
The insights and developments shared at the conference not only highlight the progress made but also set a clear direction for future research. By embracing 3D cell culture technologies and NAMs, researchers and industry professionals can look forward to more efficient, accurate and ethical preclinical testing processes, ultimately leading to better patient outcomes and groundbreaking therapeutic discoveries.
Register now for 10th Annual Conference!
References:
1. Selby M., Delosh R., Laudeman J., Ogle C., Reinhart R., Silvers T., Lawrence S., Kinders R., Parchment R., Teicher B.A.,& Evans, D. M. (2017). 3D models of the NCI60 cell lines for screening oncology compounds. SLAS DISCOVERY: Advancing Life Sciences R&D, 22(5), 473-483. https://doi.org/10.1177/2472555217697434
2. Guillemet, D., Belles, C., Gomes, A., Azalbert, V., André, M. et al. (2022). Screening for anti-adipogenic, pro-lipolytic and thermogenic plant extracts by models associating intestinal epithelial cells with human adipose cells. European journal of nutrition, 61(4), 2201–2215. https://doi.org/10.1007/s00394-021-02794-8
