GrowDex®-A Functionalized with Biotinylated Fibronectin and Vitronectin Promotes 3D Culture of Fibroblasts
Jenni Leppiniemi¹, Zeeshan Mutahir¹,², Piia Mikkonen3, Markus Nuopponen3, Paula Turkki1,4 and Vesa P. Hytönen1,4
1 Faculty of Medicine and Health Technology and BioMediTech, Tampere University, FI-33014 Tampere, Finland
2 School of Biochemistry and Biotechnology, University of the Punjab, 54590 Lahore, Pakistan
³ UPM Biomedicals, Helsinki, Finland
4 Fimlab Laboratories, Biokatu 4, FI-33520 Tampere, Finland
GRAPHICAL ABSTRACT
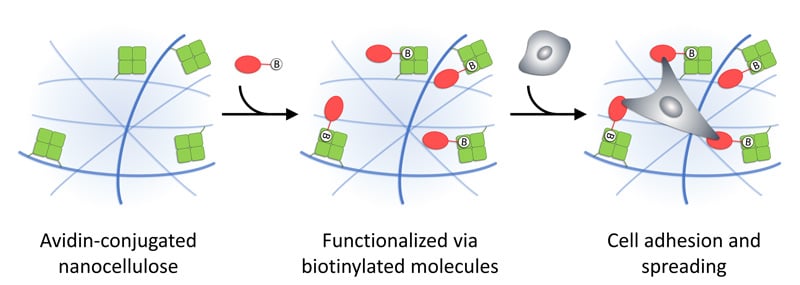
Figure 1. Illustration of functionalization of GrowDex-A via biotinylated molecules, which may provide cell adhesion sites and enable cell spreading in 3D. Reprinted (adapted) with permission from Biomacromolecules 2021, 22 (10), 4122-4137. Copyright 2021 American Chemical Society.
INTRODUCTION
Many cells are anchorage-dependent, which means that they must adhere to a substrate to survive. In human or animal body, this substrate is often ECM, a network of insoluble protein biopolymers, which contains binding sites mediating interactions with cells. However, hydrogels intended for cell encapsulation are often lacking their own binding motifs, and therefore, incorporating adhesive motifs into hydrogels may allow cell attachment to mediate cell viability, proliferation and differentiation.
GrowDex®-A is an avidin-conjugated nanofibrillar cellulose hydrogel (Figure 1). Avidin is a tetrameric protein that is capable to bind biotin or biotinylated molecules with extreme affinity and specificity. Therefore, when biotinylated molecules are mixed with GrowDex-A, they will be efficiently bound to hydrogel. Using this strategy, GrowDex-A can be customized and functionalized with different biotinylated molecules e.g. proteins or peptides, to create a cell specific matrix for 3D cell culture assays.
Here we describe how GrowDex-A can be functionalized with biotinylated proteins that provide cell adhesion motifs. We selected fibronectin (FN) and vitronectin (VN) for chemical biotinylation, as they both serve as integrin ligands and contain an RGD-sequence, which is important for integrin binding [1]. The performance of GrowDex-A functionalized with biotinylated proteins (B-FN or B-VN) was demonstrated in 3D cell culture experiments using mouse embryonic fibroblasts (MEFs). We observed a clearly enhanced cell viability, when cells were grown within GrowDex-A in the presence of the anchored biotinylated proteins compared to GrowDex-A alone. The Live/Dead staining was used to visualize cells within hydrogels.
MATERIALS
- GrowDex®-A, 1.0% (Cat No 300 103 005, UPM Biomedicals, Finland)
- Fibronectin and vitronectin proteins were purified from human plasma in house
- The mouse embryonic fibroblast (MEF) cell line was a kind gift from Dr. Wolfgang Ziegler and has been previously described by Xu et al. [2]
- CellTiter-Glo® 3D Cell Viability Assay (Cat No. G9681, Promega, USA).
- Cell culture medium: High-glucose Dulbecco’s Modified Eagle Medium (DMEM, Cat No. 61965026) supplemented with 1% (v/v) GlutaMax (Gibco™) and 10% fetal bovine serum (FBS, Gibco™, Thermo Fisher Scientific, USA)
- Ultra-low attachment 96-well plates (Cat No. 3474, Corning Life Sciences, USA)
- Glass bottom 96-well plates (Cat No. P96-1.5H-N, Cellvis, USA)
- Biotinylation reagent: Ez-Link NHS-LC-biotin (Cat No. 21336, Thermo Fisher Scientific, USA)
- Calcein, AM, cell-permeant dye (Cat No. C1430, Invitrogen, USA)
- Propidium Iodide (Cat No. BMS500PI, Thermo Fisher Scientific, USA)
- Envision multilabel plate reader (PerkinElmer, USA)
- Zeiss LSM 780 Laser Scanning Confocal Microscope (Carl Zeiss AG, Germany) using a 25×/0.80, WD 0.57 mm objective and water as immersion medium.
METHODS
Purification and biotinylation of proteins:
- Fibronectin and vitronectin were purified from human plasma. For fibronectin, gelatin affinity column chromatography was used whereas vitronectin was purified by heparin affinity chromatography. Purified proteins were dialyzed in phosphate buffered saline (PBS, pH 7) and chemically biotinylated as previously described [3]. An amine-reactive biotinylation reagent (Ez-Link NHS-LC-biotin) was dissolved in DMSO to a 10 mM concentration just before use. A 20-fold molar excess of this reagent was added to the protein solution and incubated for 30 min at room temperature (RT). After biotinylation, proteins were dialyzed in PBS (pH 7) overnight with multiple changes of dialysis buffer to remove non-reacted biotin.
Functionalization of GrowDex-A and embedding of cells:
- Stock GrowDex-A (1.0% w/v) was first gently mixed with biotinylated fibronectin (B-FN) or biotinylated vitronectin (B-VN) and incubated for an hour at RT. As controls, non-biotinylated fibronectin and vitronectin were used. After incubation, the hydrogels were diluted with cell culture medium and mixed thoroughly. Finally, cell suspension was mixed with the hydrogel by pipetting gently up and down a couple of times until the mixture appeared visually homogeneous.
- In the final cell-hydrogel mixture the concentration of GrowDex-A was 0.5% (w/v), the final concentration of biotinylated and non-biotinylated proteins [B-FN, B-VN, FN (control) and VN (control)] were 50 µg/mL, and the cell seeding density was 100 000 cells/mL.
- Working example: for 1 mL of final cell-hydrogel mixture, take 500 µL of GrowDex-A (1.0% w/v) and add 50 µL of biotinylated protein (1 mg/mL), incubate for an hour at RT. Add 250 µL of cell culture medium and finally add 200 µL of cell suspension, which are in suspension at a stock concentration of 500 000 cells/mL.
3D Cell Culture in Functionalized Hydrogel:
- 100 µL of cell-hydrogel mixture was added per well to 96-well plates.
- Glass bottom 96-well plates were used for Live/Dead analysis.
- Flat bottom ultra-low attachment 96-well plates were used for CellTiter-Glo® 3D Cell Viability Assay.
- After 30 min incubation at 37 °C, 100 µL of cell culture medium was gently added on top of hydrogel.
- Samples were incubated at 37 °C and 5% CO₂. Every second or third day the plates were centrifuged at 100 g for 5 min at RT, and half of the top layer medium was replaced with fresh cell culture medium.
Cell Viability Assay:
- The cell viability was determined on day 0, day 3 and day 7 by using a CellTiter-Glo® 3D Cell Viability Assay. The well plate was let to equilibrate to RT, then centrifuged at 200 g for 5 min, and 100 µL of medium was removed from the well and replaced with 100 µL of CellTiter-Glo® -reagent to lyse the cells. The plate was shaken for 5 minutes, centrifuged again at 200 g for 5 min and then incubated for 30 min at RT.
- The luminescence was recorded by Envision multilabel plate reader. The mean of luminescence values measured for cell-free hydrogels were subtracted as a reference value. All samples were analyzed as triplicates. The relative luminescence units (RLU) on day 3 and day 7 were normalized to the RLU measured at day 0.
Live/Dead Staining:
- Samples were Live/Dead stained using fluorescent dyes, Calcein AM (2.5 µM final concentration) and Propidium Iodide (2 µM final concentration).
- Cells were cultivated in hydrogels for three days. Then plate was centrifuged at 100 g for 5 min at RT, the medium of top of hydrogel was removed and the samples were gently washed with PBS. 100 µL of dye-PBS solution was added per well and incubated for an hour at 37 °C and 5% CO2 . As reference, the fibroblasts were grown as 2D culture on the well-plate (glass) bottom.
- Imaging and analysis: The stained samples were imaged using Zeiss LSM 780 Laser Scanning Confocal Microscope using a 25×/0.80, WD 0.57 mm objective and water as immersion medium. 488 nm and 561 nm lasers were used to excite Calcein AM and PI, and emission filters were from 493 to 582 nm for Calcein AM and from 582 to 718 nm for PI. For biofunctionalized hydrogels with cells, Z-stacks (~300 µm with interval of 1 µm) were imaged to be able to observe the growth of cells inside the hydrogel. Images were taken using Zeiss Zen Black software and analyzed using ImageJ and Huygens Essential software. Maximum intensity projections of Z-stacks were prepared. For 3D views, a 3D viewer plugin was used with volume rendering option.
RESULTS
The number of living cells increased within all hydrogels during the 7-day cultivation period, indicating that all the studied hydrogels were cytocompatible and provided suitable 3D matrix supporting cell proliferation (Figure 2a and b). Within plain GrowDex-A, the number of live cells increased 2.0 ± 0.2-fold on three days and 3.9 ± 0.4 times on seven days (Table 1). When GrowDex-A was functionalized with biotinylated adhesion proteins (B-FN and B-VN), the cell proliferation increased. For example, in the presence of B-FN the number of viable cells increased 2.2 ± 0.3-fold on three days and 6.7 ± 1.2-fold on seven days (p < 0.01, n = 3, when compared to GrowDex-A). For GrowDex-A + FN (control), the number of live cells increased 1.8 ± 0.1-fold on three days and 3.8 ± 0.6-fold on seven days, indicating the growth of cells to be very similar to GrowDex-A alone. Within GrowDex-A + B-VN, the cell proliferation was further increased; the number of live cells increased 2.5 ± 0.1-fold on three days and 9.2 ± 0.4 times on seven days (p < 0.001, n = 3, when compared to GrowDex-A). However, the proliferation of cells in the presence of vitronectin seemed to be more or less independent of adhesion, as a non-biotinylated VN control produced an effect similar to B-VN. This may reflect the differences between these integrin ligands. Adhesion on fibronectin has been found to be dependent on mechanical load applied on the integrin-FN bond, while vitronectin appears to support integrin binding during early adhesion [4]. The results obtained with GrowDex-A indicated that the adherence of cells to their surroundings supports their survival and proliferation in a 3D environment.

Figure 2. The CellTiter-Glo 3D Cell viability assay was used to determine the number of viable cells within GrowDex-A hydrogels functionalized by biotinylated fibronectin (B-FN), fibronectin (FN) control, biotinylated vitronectin (B-VN) and vitronectin (VN) control. A cell seeding density of 100 000 cells/mL was used and cell viability was determined on day 0 and after three and seven days of cultivation. (a) Relative luminescence units (RLU) of different samples. (b) The relative luminescence (RLU) values on day 3 and on day 7 of each sample were normalized by setting the luminescence values detected on day 0 to 1. Each bar represents mean value ± SD for three parallel samples. The statistical analysis was performed by comparing to GrowDex-A using one-way ANOVA and Bonferroni’s multiple comparison test, * = p < 0.05; ** = p < 0.01; *** = p < 0.001. Reprinted (adapted) with permission from Biomacromolecules 2021, 22 (10), 4122-4137. Copyright 2021 American Chemical Society.
Table 1, related to Figure 2b. The number of viable cells within GrowDex-A hydrogels functionalized by biotinylated fibronectin (B-FN), fibronectin (FN) control, biotinylated vitronectin (B-VN) and vitronectin (VN) control, and cells only as negative control, measured by the CellTiter-Glo 3D Cell viability assay. The relative luminescence (RLU) values on day 3 and on day 7 of each sample were normalized by setting the luminescence values detected on day 0 to 1
Live/Dead staining and confocal imaging was used to visually study the distribution and morphology of the MEFs cultured within different hydrogel samples (Figure 3). Most cells were alive in all tested conditions, indicating that all studied hydrogels were cytocompatible. When GrowDex-A was functionalized with biotinylated proteins, B-FN and B-VN, cells seemed to grow deeper inside the hydrogel and the morphology was more elongated than with cells cultured in hydrogel without adhesion proteins. In the presence of B-FN and B-VN, cells also seemed to form more contacts with each other. The results support the hypothesis that anchored fibronectin and vitronectin provide adhesion sites for MEFs and enhance their proliferation in 3D.

Figure 3. Confocal microscopy imaging of MEFs within GrowDex-A hydrogels (0.5% w/v) stained with Calcein-AM (green, live cells) and propidium iodide (magenta, dead cells). Cells were embedded in different hydrogels (GrowDex-A, GrowDex-A+B-FN and GrowDex-A+B-VN) using seeding density of 100 000 cells/mL and cultivated for three days. Cells only, is shown as a control sample. A differential interference contrast image of one Z-plane, a maximum intensity projection of Z-stack and a 3D view are shown for each sample. The scale bars are 100 µm. Reprinted (adapted) with permission from Biomacromolecules 2021, 22 (10), 4122-4137. Copyright 2021 American Chemical Society
REFERENCES
- Bachmann, M., Kukkurainen, S., Hytönen, V.P. and Wehrle-Haller, B. “Cell Adhesion by Integrins.” Physiological Reviews 2019, 99, 1655-1699, https://doi.org/10.1152/physrev.00036.2018.
- Xu, W., Baribault, H. and Adamson, E. D. “Vinculin knockout results in heart and brain defects during embryonic development.” Development 1998, 125 (2), 327–337, https://doi.org/10.1242/dev.125.2.327.
- Leppiniemi, J., Mutahir, Z., Dulebo, A., Mikkonen, P., Nuopponen, M., Turkki, P., and Hytönen, V.P. “Avidin-conjugated Nanofibrillar Cellulose Hydrogel Functionalized with Biotinylated Fibronectin and Vitronectin Promotes 3D Culture of Fibroblasts.” Biomacromolecules 2021: 22, 10, 4122-4137, https://doi.org/10.1021/acs.biomac.1c00579.
- Bachmann, M., Schäfer, M., Mykuliak, V. V., Ripamonti, M., Heiser, L., Weißenbruch, K., Krübel, S., Franz, C. M., Hytönen, V.P., Wehrle-Haller, B. and Bastmeyer, M. “Induction of ligand promiscuity of αVβ3 integrin by mechanical force.” J. Cell Sci. 2020, 133 (9), jcs242404, https://doi.org/10.1242/jcs.242404.