Mesenchymal Stem Cell Differentiation in GrowDex®-T
Jonathan Sheard1,2, Darius Widera2
1Sheard BioTech Ltd, 2University of Reading, UK
INTRODUCTION
Mesenchymal stem cells (MSCs) are clinically relevant and currently used in trials for medical conditions including immune rejection, neurodegenerative disease, orthopaedic issues as well as autoimmune disease [1]. Therefore, it is important to consider the culture conditions and delivery of these cells in a known and controlled manner towards regenerating damaged tissue or wound sites. Specifically, bone or cartilage forming cells in the appropriate matrix can be used to recover or replace bone fractures, damaged cartilage or critical size defects [2, 3].
MSCs are fibroblast-like cells that give rise to mesenchymal derivatives. 2D plastic adherence is a minimal criterion for MSC identification [4, 5]. However, culture expansion and differentiation of MSCs in a 3D matrix provides a more physiologically relevant model of development than when performed in 2D conditions. Indeed, it is known that the differentiation potential and growth of cells will vary depending on matrix composition [6-8],
whilst dynamic 3D culture techniques and mechanical ques have also been reported to affect
adipogenic and osteogenic differentiation properties of MSCs [9, 10]. Previously, we have shown the successful culture expansion and differentiation of MSCs in native nanofibrillar cellulose (NFC), GrowDex® [11, 12]. Here we have demonstrated the adipogenic and osteogenic differentiation of human adipose derived MSCs embedded in the anionic NFC, GrowDex®-T, as reported in Sheard, J.J. et al., (2019).
MATERIALS
• Adipose derived mesenchymal stem cells were obtained from Lonza (Cat No. PT-5006,
Slough, United Kingdom)
• Complete media: DMEM-High Glucose (Cat No. D5671-500ML, Sigma) supplemented
with 10% heat inactivated foetal bovine serum (Cat No. F9665, Lot: RNBG8272,
Sigma), 1% L-glutamine (200 mM, Cat No. G7513-100ML, Sigma), 1% penicillin with
streptomycin (10,000 units penicillin and 10 mg streptomycin per ml, Sigma).
• Differentiation Media: StemPro™ Adipogenesis or Osteogenesis Differentiation Kit (Cat
No. A1007001 and Cat No. A1007201, ThermoFisher) supplemented with 1% Pen/
Strep
• GrowDex-T 1.0% (Cat No. 200 103 005, UPM Biomedicals)
• 24 well tissue culture inserts: 3.0 μm pore PET membrane (Cat No. 83.3932.300,
Sarstedt)
• Low adhesion 24 well cell-culture plate (Cat No. 83.3922.500, Sarstedt)
• 4% Paraformaldehyde (PFA)
• Oil Red O: 300 mg Oil Red O in 100 ml 99% isopropanol (Cat No. O0625-25G, Sigma Aldrich)
• Alizarin Red S: 2 g Alizarin Red S in 100 ml distilled water, pH 4.1 with 0.1% NH4OH, filtered (Cat No. A5533-25G, Sigma Aldrich)
• EVOS Live imaging system (ThermoFisher)
METHODS
1. Adipose derived MSCs were culture expanded in 2D in complete media and incubated at 37°C with 10% CO2. Following trypsinization, cells were resuspended in complete media at a concentration of 1x106 cells/ml.
2. Stock GrowDex-T (1.0% w/v) was diluted, firstly with culture media and then with media containing MSCs, to make a final working solution with a GrowDex-T concentration of 0.2% and 5x105 cells/ml [14].
3. Working example: for 1 ml of final working solution, add 200 μl of GrowDex-T to 700 μl of complete media and mix thoroughly by pipetting whilst avoiding bubbles. Then add 100 μl of cells which are in suspension at a stock concentration of 5x105 cells/ml and mix thoroughly. This will give you a 1 ml working solution with 5x105 cells/ml in 0.2% GrowDex-T.
4. Subsequently, 100 μl of cells embedded in 0.2% GrowDex-T was then transferred to a 24-well tissue culture insert. 100 μl of complete media was gently added on top of the cells in the tissue culture insert and 1 ml of complete media was added to the outer well.
5. Medium in the outer well and in the tissue culture insert was carefully changed after 3 days to either complete media (control), or Stem Pro Adipogenic/Osteogenic media and incubated at 37°C with 10% CO2.
6. All media changes were performed slowly and gently by placing the pipette to the edge of the well above the GrowDex-T, removing 50% of the liquid and replacing it with fresh media. This was done without disturbing the GrowDex-T layer.
7. Control and differentiation media were replaced every 2 to 3 days and cells were maintained for 21 days.
8. Following the differentiation period, all media was carefully removed from the wells and tissue culture inserts, ensuring not to disturb the GrowDex-T, and cells in the tissue culture insert were washed once with PBS for 5 mins and then fixed with 4% PFA for 30 mins. This was performed by gently adding the PBS/PFA incubating for the required time, then removing the solution without disturbing the GrowDex-T.
9. For Oil Red O staining: 3 parts of the stock solution was mixed with 2 parts ddH2O and filtered. Following fixation, cells in the tissue culture inserts were washed once with sterile ddH2O for 5 mins. 100 μl of staining solution was added to the cells for 5 mins.
10. For Alizarin red staining: following fixation, 100 μl of Alizarin red staining solution was added to cells in the tissue culture inserts and incubated for 45 mins at RT in the dark.
11. The staining solutions were removed, and unbound dye was washed off by 3 to 5 washing steps with ddH2O for 5 mins each.
12. Images were taken using the EVOS imaging system.
RESULTS
Following 21 days of differentiation treatments, adipose derived MSCs showed lipid accumulation (Fig. 1) and strong calcium deposition (Fig. 2) when compared to control untreated cells. Oil Red O staining of cells treated with adipogenic differentiation media revealed strong staining of intracellular lipids (Fig. 1B and C, white arrows) in contrast to the control untreated cells (Fig. 1A).Following 21 days of differentiation treatments, adipose derived MSCs showed lipid accumulation (Fig. 1) and strong calcium deposition (Fig. 2) when compared to control untreated cells. Oil Red O staining of cells treated with adipogenic differentiation media revealed strong staining of intracellular lipids (Fig. 1B and C, white arrows) in contrast to the control untreated cells (Fig. 1A).
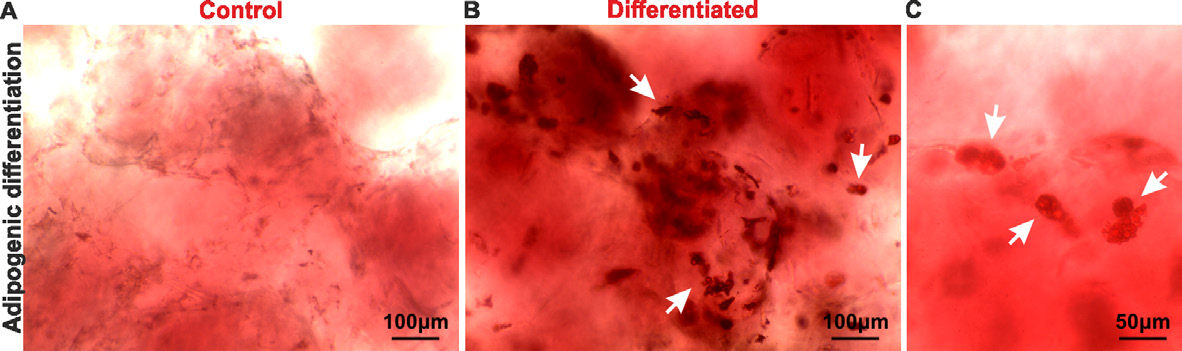
Figure 1. Bright field images showing MSCs stained with Oil Red O following 21 days of incubation in either control (A) or adipogenic differentiation (B-C) media. Deposition of intracellular lipid droplets can be seen in differentiated cells (white arrows). Bar: 200 μm (A-B), 50 μm (C).
Alizarin red staining of cells cultivated in osteogenic differentiation media showed high levels of calcium deposition (Fig. 2B) compared to the control (Fig. 2A).

Figure 2. Images showing Alizarin Red S staining of MSCs cultured in GrowDex-T for 21 days in either control (A) or osteogenic differentiation (B) media. Pixel intensity from images collected show significantly darker staining of osteogenic differentiation treated cells compared to control (C). ***P<0.001. Bar: 500μm.
CONCLUSIONS
Here, we demonstrated that adipose derived MSCs cultured in 0.2% GrowDex-T can be driven along both adipogenic and osteogenic differentiation lineages. The anionic NFC, GrowDex-T has tuneable viscosity and is biocompatible with human cells. The morphology of MSCs in GrowDex-T were stellate and fibroblastic, in comparison to the a spheroid morphology observed in GrowDex that is native nanofibrillar cellulose [11, 12, 15].
Adipogenic or osteogenic differentiation potential is an important identification criterion for MSCs [4, 5]. Compared to 2D culture conditions, differentiation of MSCs in a 3D microenvironment represents a more physiological approach and is known to enhance the levels of adipogenesis and osteogenesis [8-10]. Additionally, MSCs embedded in 3D scaffolds show greater regenerative capacity [2].
Since GrowDex-T is an animal free matrix, it has great potential for clinical applications. Overall, it provides a promising tool in studying MSCs in 3D for new model development or treatment strategies such as the regeneration of bone injuries or critical size defects.
REFERENCES
1. Naji, A., et al. (2019). "Biological functions of mesenchymal stem cells and clinical implications." Cellular and Molecular Life Sciences 76(17): p. 3323-3348.
2. Bajada, S., et al. (2007). "Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation." The Journal of Bone and Joint Surgery. British volume 89-B(10): p. 1382-1386.
3. Reznikov, N., et al. (2016). "A materials science vision of extracellular matrix mineralization." Nature Reviews Materials 1: p. 16041.
4. Pittenger, M.F., et al. (1999). "Multilineage potential of adult human mesenchymal stem cells." Science 284(5411): p. 143-7.
5. Dominici, M., et al. (2006). "Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement." Cytotherapy 8(4): p. 315-7.
6. Awad, H.A., et al. (2004). "Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds." Biomaterials 25(16): p. 3211-3222.
7. Jäger, M., et al. (2005). "Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells Cultured onto Three Different Polymers In Vitro." Annals of Biomedical Engineering 33(10): p. 1319-1332.
8. Jung, J.P., et al. (2016). "Heterogeneous Differentiation of Human Mesenchymal Stem Cells in 3D Extracellular Matrix Composites." BioResearch Open Access 5(1): p. 37-48.
9. Frith, J.E., et al. (2010). "Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential." Tissue Eng Part C Methods 16(4): p. 735-49.
10. Lo, Y.-P., et al. (2016). "Three-dimensional spherical spatial boundary conditions differentially regulate osteogenic differentiation of mesenchymal stromal cells." Scientific Reports 6: p. 21253.
11. Azoidis, I., et al. (2017). "Three-dimensional cell culture of human mesenchymal stem cells in nanofibrillar cellulose hydrogels." MRS Communications 7(3): p. 458-465.
12. Sheard, J. "Adipose derived mesenchymal stem cells: Investigating sphere formation in relation to seeding density and hydrogel concentration." GrowDex Application Note 22.
13. Sheard, J.J., et al. (2019). "Optically Transparent Anionic Nanofibrillar Cellulose Is Cytocompatible with Human Adipose Tissue-Derived Stem Cells and Allows Simple Imaging in 3D." Stem Cells International 2019: p. 12.
14. UPM-Biomedicals "Recommended Procedure For Diluting and Mixing GrowDex®-T." GrowDex-T Application Note 1.
15. Sheard, J. "Hanging drop culture of mesenchymal stem cells within nanofibrillar cellulose." GrowDex Application Note 23.